#gmp
ليس لديك مجموعات تناسب بحثك
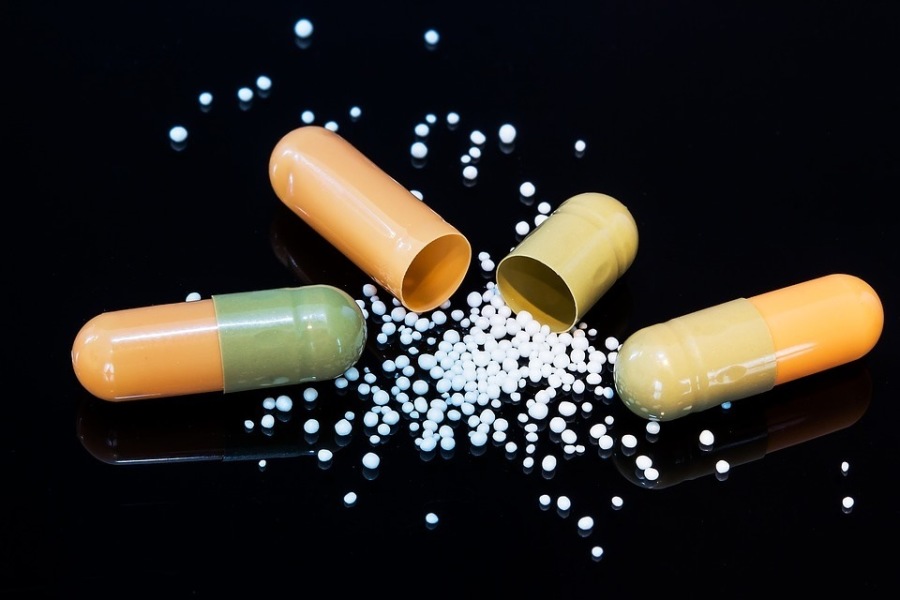
Foundations of GMP: Active Pharmaceutical Ingredient (API)
Mohammed Abdul Jawad · FREE Self-Paced eLearning - Download your FREE 'Certificate of Completion' upon successful completion of Self-Paced eLearning. · CLICK HERE TO REQUEST ENROLLMENT for this eLearning. · Course Description This eLearning course aims to improve your knowledge and understanding of ...

Foundations of GMP: Water Systems
Mohammed Abdul Jawad · FREE Self-Paced eLearning - · Download your FREE 'Certificate of Completion' · upon successful completion of Self-Paced eLearning. · CLICK HERE TO REQUEST ENROLLMENT for this eLearning. · Course Description This eLearning course aims to improve your knowledge and understanding o ...

Foundations of GMP: Heating, Ventilation, and Air Conditioning (HVAC) Systems
Mohammed Abdul Jawad · FREE Self-Paced eLearning - Download your FREE 'Certificate of Completion' upon successful completion of Self-Paced eLearning. · CLICK HERE TO REQUEST ENROLLMENT for this eLearning. · Course Description This eLearning course aims to improve your knowledge and understanding of ...

Foundations of GMP: Data Integrity and Computer System Validation
Mohammed Abdul Jawad · FREE Self-Paced eLearning - Download your FREE 'Certificate of Completion' upon successful completion of Self-Paced eLearning. · CLICK HERE TO REQUEST ENROLLMENT for this eLearning. · Course Description This eLearning course aims to improve your knowledge and understanding of ...

Foundations of GMP: Final Dosage Form
Mohammed Abdul Jawad · FREE Self-Paced eLearning - Download your FREE 'Certificate of Completion' upon successful completion of Self-Paced eLearning. · CLICK HERE TO REQUEST ENROLLMENT for this eLearning. · Course Description This eLearning course aims to improve your knowledge and understanding of ...

Foundations of GMP: Deviations, Root Cause Analysis (RCA) Tools and Corrective and Preventive Action (CAPA)
Mohammed Abdul Jawad · FREE Self-Paced eLearning - · Download your FREE 'Certificate of Completion' · upon successful completion of Self-Paced eLearning. · Foundations of GMP: Deviations, Root Cause Analysis (RCA) Tools and Corrective and Preventive Action (CAPA) - Sequence flow for handling of any n ...

Foundations of GMP: Quality Risk Management
Mohammed Abdul Jawad · FREE Self-Paced eLearning · Download your FREE 'Certificate of Completion' upon successful completion of Self-Paced eLearning. · CLICK HERE TO REQUEST ENROLLMENT for this eLearning. · Course Description This eLearning course aims to improve your knowledge and understanding of ...

Foundations of GMP: Validation
Mohammed Abdul Jawad · FREE Self-Paced eLearning · Download your FREE 'Certificate of Completion' upon successful completion of Self-Paced eLearning. · CLICK HERE TO REQUEST ENROLLMENT for this eLearning. · Course Description This eLearning course aims to improve your knowledge and understanding of ...

When Pharma Lacks Data Integrity
Mohammed Abdul Jawad · It’s often deviant discrepancies, poor procedures or deficient data that can cause a pharma company to commit lapses of varying levels. In a rarity, when there’s corrupt culture, then there are chances that possible data integrity violations, during internal audit processes, will ...
قد تكون مهتمًا بهذه الوظائف
-

Inbound Logistics
تم العثور عليها في: beBee S2 SA - منذ 13 ساعة
Roche Johannesburg, المملكة العربية السعودية دوام كاملKey Responsibilities · Inventory Management · Monitor inventory system variances by drawing system reports, comparing with service provider reports, identifying variances, investigating reasons and implementing corrective action to reconcile stock levels. · Resolve outstanding ou ...
-
Structure Technician
تم العثور عليها في: Talent SA A C2 - منذ أسبوع
Eram Talent Dammam, المملكة العربية السعوديةUnder the line management of thetechnical direction of the Structures Maintenance Supervisor, beresponsible for all activities relating to earthworks (embankments& cuttings), Bridges, Culverts, Retaining Walls and SupportStructures for operational equipment · Toundertake technica ...
-

مطلوب شيف مكة المكرمة السعودية
تم العثور عليها في: beBee S2 SA - منذ 6 أيام
ابو نادر الحمراء, المملكة العربية السعودية فريلانسرطباخ يمني محترم · محترف طباخ جميع لرز خبره 15اسنه طباخ جميع لحم مفطاحات حاشي مفطح عزائم اعرس ...

