Cleaning Validation in Pharma
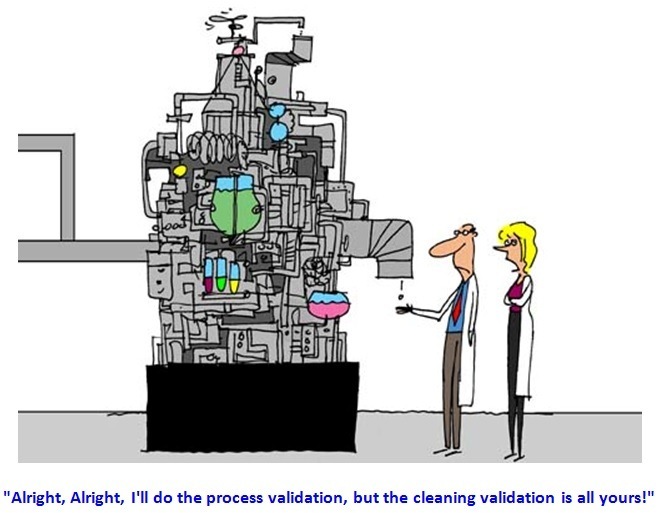
Any non-compliance towards cleaning validation norms means there’s ineffective cleaning validation that poses potential risk to patient safety and product quality due to cross contamination during pharmaceutical production. When inspected, observed and caught by regulatory authority, there comes a warning letter.
- Failure to determine and adhere to correct written procedures that are necessary to prevent contamination of drug products.
- Failure to maintain floors, walls and ceilings of smooth, hard surfaces in the production, processing, packing or holding of a drug product in a clean and sanitary condition.
- Failure to guarantee that production personnel wear clothes appropriate to protect drug product from contamination.
- Failure to establish a sufficient system for checking environmental conditions in processing area.
- Failure to establish a plentiful air supply filtered through high-efficiency particulate air filters under positive pressure in the processing areas.
- Failure to establish an ample system for cleaning and disinfecting a room and equipment to produce aseptic conditions.
- Failure to totally probe any unsolved inconsistency or failure of a batch or any of its components to meet any of its requirements whether or not the batch has already been distributed
- Failure to ascertain scientifically good and right requirements, paradigms, sampling plans, and test procedures considered to authenticate that drug products match to proper standards of identity, strength, quality and purity.
- Failure to regularly calibrate, look over or verify according to a written program designed to assure proper performance and to maintain sufficient written records of calibration checks and inspections of automatic, mechanical, or electronic equipment, including computers, used in the manufacture, processing, packing and holding of a drug product.
- Failure to set up proper controls over computers and linked systems to guarantee that changes in master production and control documentation or other records are brought about only by authorized personnel.
- Failure to apply ample procedures to avoid contamination and lack of records to show that suitable line clearance and cleaning is performed following occurrence of contamination
- Failure to have adequate written procedures for production and process controls designed to assure that respective drug products have the identity, strength, quality and/or purity they claim or are represented to possess.
- Failure to carry out lab testing of APIs to ensure conformance to specifications and to correctly report results on Certificates of Analysis (CoA).
It’s paramount for a pharma company to be concerned about patient safety, and every responsible person from respective departments should understand the regulations, rules and instructions, and play a vital role to see how the cleaning validation and continuous cleaning verification can be instigated.
Though, cleaning validation, being necessary and time consuming part of manufacturing pharmaceuticals, represents a real challenge to the pharma industry because it’s not just a matter of regulatory compliance, but what aspects concern most are the safety of pharmaceuticals, achievability and efficiency.
As a cleaning validation specialist your focus should be to lower any downtime in production and so once all important contact areas and equipment have been tested, with a wide range of techniques, you will need quick and strong analysis that is adequately specific and sensitive to spot contaminants at the acceptable residue levels (ARL) for a predetermined level of cleanliness.
To be a specialist in cleaning validation activities, it’s not only qualification that matters, but a person must have professional experiences in the pharma and biotech industries, with proficient knowledge of validation documentation in relation to equipment and facility cleaning, continuous improvement and troubleshooting processes.
What’s the use when a person is both qualified and experienced in pharma industry but he is incapable to design, implement and review protocols and reports for cleaning development and validation, carry out investigation of deviations or discrepancies related to cleaning validation and manage and reassess validation area processes?Image source: pharmamanufacturing.com - Caption by Vic Jewell
مقالات من Mohammed Abdul Jawad
عرض المدونة
Ah, you have departed from this world so soon · but then, we accept this was your writ destiny · May ...

Understanding Stigma: A free online course for healthcare providers and other frontline clinicians · ...

FREE WEBINAR - AVAILABLE ON DEMAND · Title: Floor Level Contamination in Cleanrooms and Controlled E ...
قد تكون مهتمًا بهذه الوظائف
-
مدير دعم الأعمال
تم العثور عليها في: Talent SA A C2 - منذ يومين
Accor Hotels Al Qaisumah, المملكة العربية السعوديةشهادة جامعية في مجال ذي صلة، مثلإدارة الأعمال، أو تكنولوجيا المعلومات، أو الهندسةالصناعية. · مهارات تحليلية ممتازة وقدرة على فهمالعمليات التجارية وتحليلها. · خبرة في إدارةوتطوير أنظمة الدعم التقني والعمليات الأساسيةللأعمال. · معرفة عميقة بأنظمة تكنولوجيا المعلوماتوالبرمجيات المست ...
-
تحليلات البيانات
تم العثور عليها في: DrJobAR SA A2 - منذ 5 أيام
Persistence Recruitment الرياض, المملكة العربية السعوديةقيادة المشروع: قيادة التسليم الشامل لمشاريع منصة إدارة البيانات، مما يضمن إكمالها بنجاح ضمن النطاق والجدول الزمني والميزانية إدارة أصحاب المصلحة: التعاون بشكل وثيق مع أصحاب المصلحة في المشروع معًا للمتطلبات، وتوفير التحديثات، والتأكد من التوافق بين التسليم الفني وأهداف العمل قياد ...
-
مدير الخدمات الناعمة
تم العثور عليها في: DrJobAR SA A2 - منذ 4 أيام
Alfanar saudi جازان, المملكة العربية السعوديةدرجة البكالوريوس في إدارة الأعمال، علوم الحاسوب، أو مجال ذي صلة. · فهم عميق للعمليات الأساسية في مجال الخدمات الناعمة مثل خدمة العملاء وإدارة العلاقات مع العملاء. · مهارات القيادة والإدارة لإدارة الفرق وتوجيهها نحو تحقيق الأهداف. · القدرة على تطوير استراتيجيات وخطط لتحسين تقديم ا ...


التعليقات